Every time you pick up a prescription for a generic drug, you’re holding a product that’s been engineered to match a brand-name medicine down to the last molecule - but at a fraction of the cost. The question isn’t whether it works. It’s how it’s made to work just as well, without the same price tag.
The Blueprint: Reverse Engineering the Brand-Name Drug
The journey of a generic drug starts long before any pills are pressed. Manufacturers begin by studying the original brand-name product, called the Reference Listed Drug (RLD). This isn’t just reading the label. It’s full chemical and physical reverse engineering. They analyze the active ingredient’s molecular structure, how it dissolves, how it’s absorbed, and even the exact type of filler or coating used. This step is critical because even small differences in excipients - the inactive ingredients like lactose or cellulose - can change how quickly the drug enters your bloodstream. For example, if the brand-name version uses a specific grade of lactose with a certain particle size, the generic maker must find an equivalent. A 2024 Reddit post from a pharmaceutical engineer with over a decade of experience described how a simple switch in lactose supplier once caused an entire batch of tablets to fail dissolution tests. The hardness was fine, but the drug didn’t release properly. That’s why every raw material is tracked, tested, and documented.Designing for Consistency: Quality by Design (QbD)
Modern generic drug manufacturing follows the Quality by Design (QbD) framework, a global standard set by the International Council for Harmonisation (ICH). This means manufacturers don’t just test the final product - they design the entire process to guarantee quality from the start. They identify three key factors:- Critical Quality Attributes (CQAs): What must the drug do? For example, it must release 85% of its active ingredient within 30 minutes.
- Critical Material Attributes (CMAs): What properties of the raw materials matter? Like the flow rate of the powder or moisture content of the filler.
- Critical Process Parameters (CPPs): What steps in manufacturing must be tightly controlled? Temperature, mixing time, compression pressure.
The Seven-Step Manufacturing Process
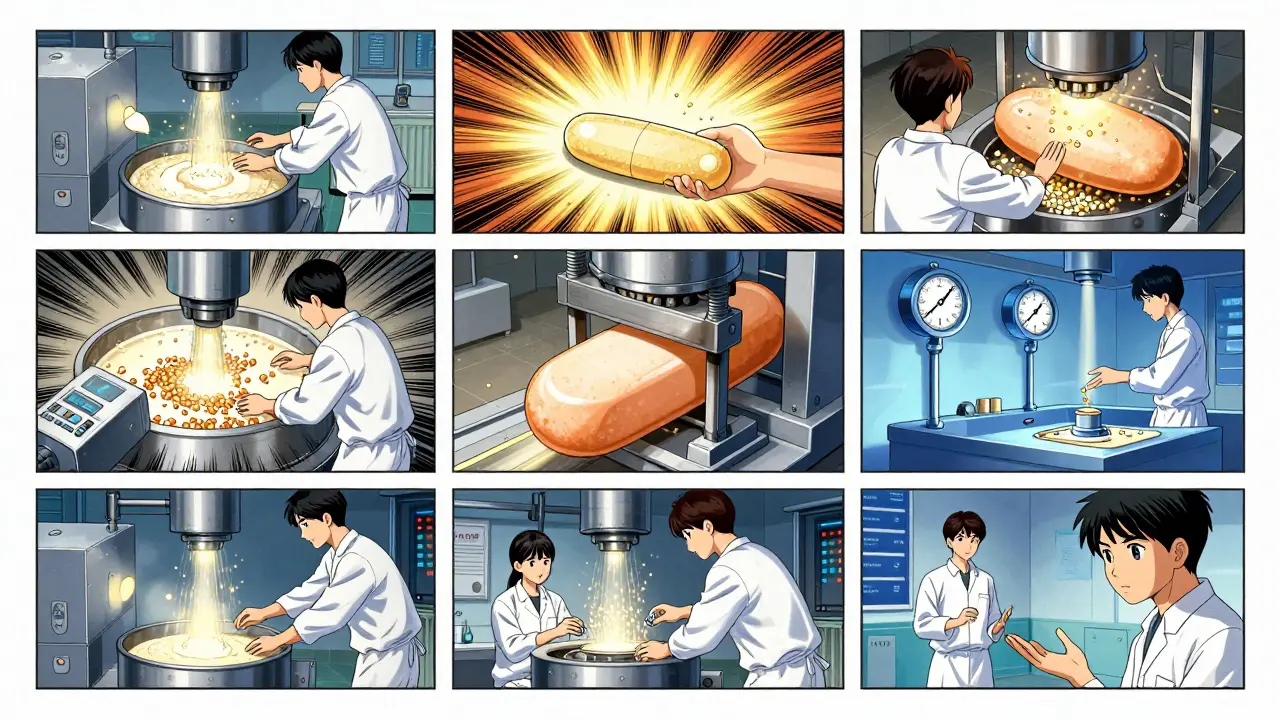
Once the formula is locked in, production begins. Here’s how a typical generic tablet is made:- Formulation: The active pharmaceutical ingredient (API) is blended with excipients - binders, disintegrants, lubricants - in precise ratios. This mixture is called the powder blend.
- Mixing and Granulation: The powder is either mixed dry or turned into wet granules using water or alcohol. Granulation ensures the powder flows evenly into the tablet press and doesn’t separate during handling.
- Drying: If wet granulation was used, the granules are dried in ovens at controlled temperatures. Too much moisture? The tablets could crumble. Too little? They might not bind properly.
- Compression and Encapsulation: Dry granules are pressed into tablets using high-force machines. For capsules, the powder is filled into gelatin or plant-based shells. Each tablet must weigh within ±5% of the target for those under 130mg, or ±7.5% for heavier ones - a strict FDA rule.
- Coating: Many tablets get a thin film coating. It masks bitter tastes, protects the drug from moisture, and sometimes controls how fast it dissolves. A delayed-release pill might have a coating that only breaks down in the intestine, not the stomach.
- Quality Control: At every stage, samples are pulled. Tablets are checked for weight, hardness, thickness, and dissolution. A dissolution test simulates how the drug breaks down in the body. The generic must release the same amount of active ingredient at the same rate as the brand-name version - within 80% to 125% of the original’s performance.
- Packaging and Labeling: Tablets are sealed in blister packs or bottles with child-resistant caps. Labels must list the generic name, strength, manufacturer, and expiration date - but cannot copy the brand’s color or shape. U.S. trademark law requires generics to look different, even if they work the same.
Regulatory Approval: The ANDA Pathway
You can’t sell a generic drug just because you made it. You need FDA approval through the Abbreviated New Drug Application (ANDA). This is the secret to why generics cost so much less. Unlike brand-name drugs, which require years of expensive clinical trials to prove safety and effectiveness, generics rely on the original drug’s data. The ANDA process skips those trials and focuses on proving one thing: bioequivalence. To do this, manufacturers run studies with 24 to 36 healthy volunteers. Blood samples are taken over hours to measure how much of the drug enters the bloodstream (Cmax) and how long it stays there (AUC). The generic’s numbers must fall within 80%-125% of the brand-name drug’s, with 90% confidence. That’s not a guess - it’s a statistically validated match. The FDA reviews the ANDA in about 17 months on average. But complex drugs - like inhalers, topical creams, or extended-release pills - can take up to three years. That’s because their delivery systems are harder to copy. A generic version of a nasal spray, for example, must match the spray pattern, droplet size, and lung deposition - not just the chemical content.Manufacturing Standards: CGMP and Cleanrooms
Every facility making generic drugs must follow Current Good Manufacturing Practices (CGMP). These aren’t suggestions. They’re enforceable rules. Production areas are classified as cleanrooms - from ISO Class 8 (like a hospital ward) to ISO Class 5 (like a surgical suite). Air is filtered, humidity is kept between 45% and 65%, and temperature is held at 20-25°C. Workers wear gowns, masks, and gloves. Every machine is calibrated. Every batch is traced. The FDA inspects these facilities regularly. In 2023, the most common violations were:- Failure to properly investigate out-of-specification results (37% of warning letters)
- Inadequate process validation (29%)
- Weak oversight by the quality unit (24%)
Why Some Generics Are Harder to Make Than Others
Not all generics are created equal. Simple pills - like metformin or lisinopril - are easy to copy. Hundreds of companies make them. Prices drop fast. You can buy 30 tablets of generic atorvastatin for under $5. But complex generics? Those are a different story. Take Clobetasol Propionate cream - a potent topical steroid. One manufacturer spent seven years and $47 million trying to match the skin absorption rate of the brand-name version. Even tiny differences in the cream’s base affected how deeply the drug penetrated. Traditional tests couldn’t predict that. It took real human skin studies to get it right. The same goes for inhalers, injectables, and eye drops. These require advanced engineering. That’s why only 2-5 companies make complex generics, and they charge more. Their margins are higher because they’re harder to copy.






Anjula Jyala January 27, 2026
The QbD framework is non-negotiable in modern pharma but most people don't realize how much of it is just statistical noise dressed up as science
CMAs and CPPs are just fancy terms for trial and error with a compliance checklist
That 80-125% bioequivalence window? That's a 45% variance in absorption
That's not equivalence that's a gamble
And don't get me started on the excipient substitutions
Lactose particle size changes alone can tank bioavailability
Manufacturers don't test every batch like they claim
They test one batch and extrapolate
The FDA inspections are theater
Most violations are caught by whistleblowers not auditors
And the ANDA process? It's a backdoor for Indian and Chinese labs to bypass real R&D
You think you're saving money but you're just outsourcing risk
And the color rule? That's not about trademarks that's about hiding the fact that the pills are made in different factories
It's all smoke and mirrors
Murphy Game January 27, 2026
They don't tell you that 78% of API comes from China
That means the entire US generic supply chain depends on a single authoritarian regime
What happens when the next geopolitical crisis hits
Or when they decide to weaponize medicine
And the FDA inspects these plants once every 5 years
Meanwhile every single pill you take could be contaminated
Remember the heparin scandal
Or the nitrosamine recalls
This isn't healthcare this is a national security vulnerability
And no one talks about it because the system is rigged
Big Pharma owns the regulators
They wrote the ANDA rules
You're not getting cheap medicine
You're getting a time bomb wrapped in a white tablet
John O'Brien January 27, 2026
Bro the whole generic system is actually insane in the best way
You think you're getting a $5 pill but you're really getting a $500 science experiment that passed 17 different lab tests
That tablet went through more QA than your iPhone
They test dissolution rates like it's a rocket engine
And the coating? That's not just flavoring that's a timed-release algorithm
People act like generics are knockoffs
Nah they're reverse-engineered masterpieces
The fact that you can buy metformin cheaper than a coffee and it works the same
That's not a flaw that's a triumph
And yeah the supply chain is sketchy
But so is your phone battery
At least the FDA still checks the pills
Most people don't even know what CGMP stands for
And yet they trust their meds
That's the real miracle