When you pick up a prescription, you might not think about whether the pill in your hand is the brand-name version or a generic. But behind that simple swap is a tangled web of state laws that dictate whether a pharmacist can make the change - and whether you even get a say. These rules aren’t the same in every state. In one place, the pharmacist substitutes automatically. In another, they need your written permission. And in a few, they can’t substitute at all for certain drugs, no matter how much money it saves.
Why Do These Laws Even Exist?
Generic drugs are chemically identical to brand-name drugs. They work the same way. They’re just cheaper - often 80% to 85% cheaper. Since 2009, generic substitution has saved the U.S. healthcare system over $1.7 trillion. That’s not small change. States created substitution laws to make sure those savings actually reach patients, especially those on Medicaid or without insurance. But they also had to protect people from risks. Some drugs, called narrow therapeutic index (NTI) drugs, have very little room for error. A tiny difference in dose - even one that’s still considered "therapeutically equivalent" - can cause serious side effects. Warfarin, levothyroxine, and certain seizure medications fall into this category. A patient switching from one brand to another generic, or between generics, might have a blood clot or a seizure. That’s why states didn’t just copy the federal rules. They built their own guardrails.Four Ways State Laws Differ - And What It Means for You
State laws on generic substitution break down into four key areas. Knowing these helps you understand why your pharmacist acts differently depending on where you live.1. Mandatory vs. Permissive Substitution
In 22 states, pharmacists are required by law to substitute a generic drug unless the doctor or patient says no. That’s mandatory substitution. In the other 28 states and Washington, D.C., substitution is optional. The pharmacist can do it - but doesn’t have to. They might wait for you to ask. Or they might just hand you the brand name if it’s in stock. This isn’t just paperwork. It affects your wallet. States with mandatory substitution have generic fill rates of 94.1%. In permissive states, it’s only 88.3%. That’s a 6-point gap - and it adds up over millions of prescriptions.2. Patient Consent: Presumed vs. Explicit
Even in mandatory states, you might not know a substitution happened until you get home. That’s because 32 states use "presumed consent." That means the pharmacist assumes you’re okay with the switch unless you say otherwise. In 18 states, you have to give explicit consent - either by signing a form, verbally agreeing, or checking a box on the screen at the pharmacy counter. Think about this: if you live near a state border, you might get different treatment for the same prescription. A patient in New York has to say yes. A neighbor in New Jersey? The generic is already in their bag. No one told them. That confusion is real. Pharmacists report patients coming back angry because they thought they were getting the brand name.3. Notification Requirements
Forty-one states require pharmacists to notify patients after a substitution. That notification might be a printed slip, a phone call, or a message on your pharmacy app. But in nine states? No notification is required. You might never know you got a different drug - unless you check the label yourself. That’s risky. If you’ve had a bad reaction to a specific generic brand before, you need to know what you’re getting. Without notification, you lose that control.4. Liability Protection for Pharmacists
If a substitution causes harm, who’s responsible? In 37 states, pharmacists are protected from lawsuits as long as they follow the state’s rules. That means if you’re given the wrong generic because the pharmacist followed the law - but you still had a bad reaction - they likely can’t be sued. That’s meant to encourage substitution. But it also means patients have fewer legal options if something goes wrong.What Happens With Special Drugs? Biosimilars and NTI Medications
The rules get even more complicated with newer drugs. Biosimilars - cheaper versions of biologic drugs like Humira or Enbrel - are treated differently. As of 2023, 49 states and D.C. have laws for them. But Hawaii is an outlier. For antiepileptic drugs, they require both the doctor’s permission and your written consent. That’s stricter than anywhere else. For NTI drugs, 15 states have extra lists. Kentucky bans substitution for digitalis and seizure meds. Minnesota has documented cases where warfarin substitutions led to dangerous bleeding. The FDA says generics are equivalent. But doctors and patients know: in real life, some people react differently. That’s why many patients with rare diseases or chronic conditions ask their doctors to write "Dispense As Written" on the prescription. That legally blocks substitution - even in mandatory states.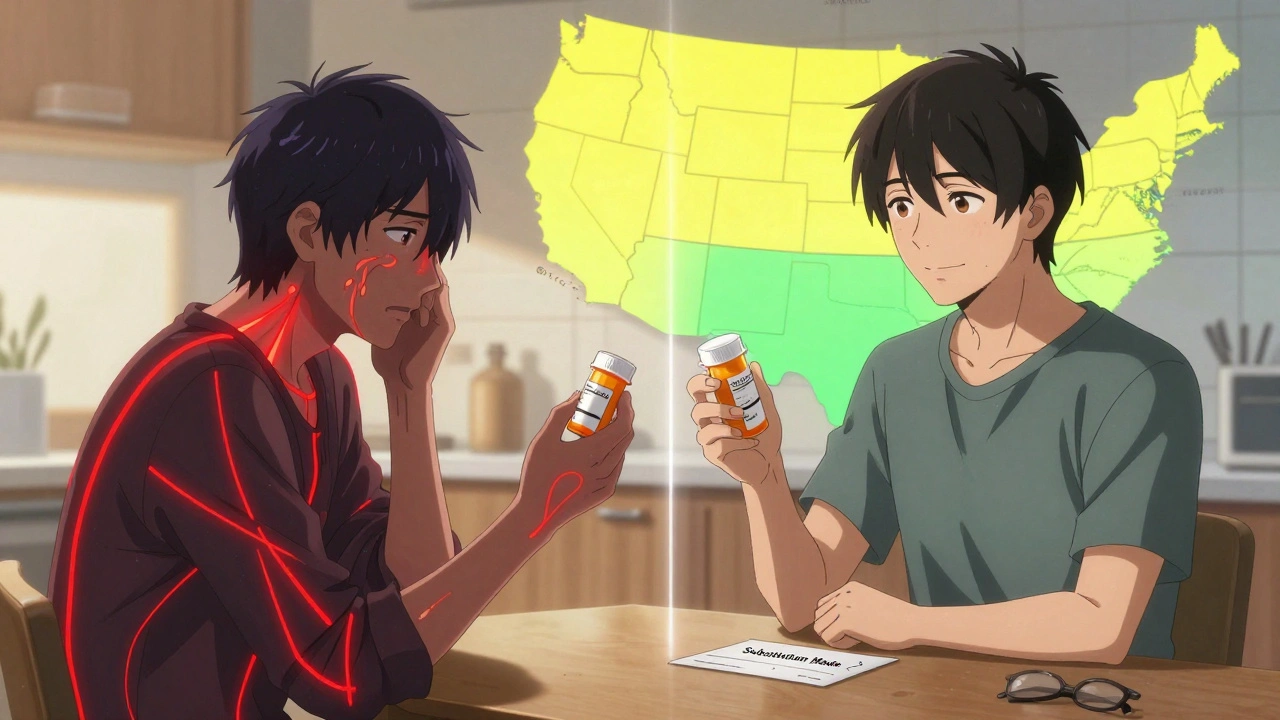
How Pharmacists Handle the Chaos
Imagine you’re a pharmacist. You fill 50 prescriptions a day. Ten of them are from out-of-state prescribers. Each one has different rules. One says you can substitute unless the patient objects. Another says you must notify. A third says you can’t substitute any NTI drug - even if it’s on the FDA’s Orange Book. On average, pharmacists spend 12.7 minutes per prescription just checking state laws. That’s not time spent counseling patients. It’s time spent flipping through digital manuals, updating software, and double-checking. Most pharmacies now use automated systems that pull state rules in real time. These tools cut substitution errors by 64%. But they’re not perfect. A 2022 survey found 78% of pharmacists feel confused when handling prescriptions from different states. And 18.3% of chain pharmacy transactions involve cross-border prescriptions - making mistakes more likely.The Real Cost - And the Real Savings
The savings are massive. Generic drugs make up 92.5% of all prescriptions filled in the U.S. That’s over $313 billion saved every year. States with strong substitution laws save even more. Medicaid programs in 27 states saved $1.2 billion annually just from mandatory substitution. But the cost isn’t just financial. It’s in patient trust. Between 2020 and 2022, the FDA received 217 reports from patients who felt their medication stopped working after a generic switch. Most involved levothyroxine or warfarin. One Reddit user wrote: "I’ve been on the same generic for years. Then my pharmacy switched it. I felt like I was drowning. I had to go to the ER. No one told me it changed." Meanwhile, 41% of cancer patients surveyed by the Life Raft Group said they worry about substitution of NTI drugs. And 28% said their doctors specifically told them to avoid it.
What’s Next? Toward Standardization - Or More Chaos?
The system is unsustainable. A 2023 draft from the Uniform Law Commission aims to create a national model for biosimilar substitution. It’s meant to replace 49 different state laws with one clear standard. But not everyone agrees. Some states want more flexibility. Rural areas with fewer specialists argue they need tighter rules. Urban areas with high generic use want faster substitution. Patient advocacy groups fear standardization will hurt people with rare diseases. Pharmacy groups say the patchwork is too confusing to manage. The Congressional Budget Office estimates that if all states aligned their laws, the U.S. could save another $8.7 billion by 2028. But that’s only if the changes don’t cause more harm than good.What You Can Do
You don’t have to be a passive player in this system. Here’s how to take control:- Always check the label. If the name or color changed, ask why.
- If you’ve had side effects from a generic before, ask your doctor to write "Dispense As Written" on your prescription.
- Know your state’s rules. A quick search for "[your state] generic substitution law" will tell you if consent is required.
- Ask your pharmacist: "Was this a substitution?" If they say yes, ask if it’s the same one you got last time.
- If you feel something’s wrong after a switch - even if it’s subtle - report it to the FDA’s MedWatch system.
Can my pharmacist substitute a generic drug without telling me?
In 32 states, yes - if the law allows substitution and you haven’t objected. These states use "presumed consent," meaning the pharmacist assumes you’re okay with the switch unless you say otherwise. In 18 states, you must give explicit consent before a substitution happens. Always check your prescription label - if the name or manufacturer changed, you were substituted.
Are generic drugs always as safe as brand-name drugs?
The FDA says yes - all generics must meet the same standards for strength, purity, and effectiveness. But for narrow therapeutic index (NTI) drugs like warfarin, levothyroxine, and seizure medications, even small differences can matter. Some patients report changes in how they feel after switching. That’s why 15 states ban substitution for certain NTI drugs, and why doctors often write "Dispense As Written" for these prescriptions.
Why do some states block generic substitution for certain drugs?
States restrict substitution for drugs with a narrow therapeutic index (NTI) because the difference between an effective dose and a harmful one is very small. For example, Kentucky bans substitution for antiepileptic drugs and digitalis. Minnesota has documented adverse events after warfarin substitutions. These rules exist to prevent rare but serious side effects - even if the FDA rates the generics as equivalent.
Can I refuse a generic drug even if my state has mandatory substitution?
Yes. Even in states with mandatory substitution, you can refuse. You can also ask your doctor to write "Dispense As Written" or "Do Not Substitute" on your prescription. That legally prevents the pharmacist from switching the drug - no matter what the state law says. Your right to choose your medication always overrides pharmacy policy.
How do I find out what my state’s generic substitution laws are?
Search online for "[your state] generic substitution law" or visit your state’s Board of Pharmacy website. The National Conference of State Legislatures (NCSL) also maintains a public database of state laws on drug substitution. Most states have clear summaries online. If you’re unsure, ask your pharmacist - they’re required to know the rules.





Elliot Barrett December 10, 2025
Why do we even have 50 different rulebooks for this? It's 2024, not 1924. Just make one national standard already.
Anna Roh December 12, 2025
I switched generics last month and got hit with severe vertigo. No one told me. My pharmacist said it was 'within FDA guidelines.' Yeah, right. My body didn't get the memo.
Katherine Rodgers December 14, 2025
Oh wow. So the system is 'complex' and 'changing.' Groundbreaking. I'm shocked the FDA and Big Pharma aren't just handing out gold stars for this circus.
Sarah Gray December 16, 2025
It's amusing how laypeople assume 'chemically identical' means 'biologically identical.' The FDA's standards are a joke. If you're on levothyroxine, you're playing Russian roulette with your metabolism.
William Umstattd December 16, 2025
My grandma in Ohio got switched to a generic warfarin and ended up in the ER. The pharmacist didn't even tell her. She thought the pill was just a different color. Now she refuses all generics. Good for her.
Katherine Chan December 16, 2025
Just ask your doc to write DAW 1 on the script and you're golden. Seriously. It's one line. You're worth the extra $10. Don't let the system gaslight you into thinking you're being cheap when you're being smart.
om guru December 18, 2025
Generic substitution is a triumph of rational economics over bureaucratic inertia. The savings are undeniable. Patient concerns must be addressed with data not fear. The system works if we trust science and streamline policy.
Nikhil Pattni December 18, 2025
Bro I looked up my state's law and it's wild. In India we have one rule: if it's cheaper and the bottle says the same thing, you take it. Here? You need a PhD in pharmacy law just to get your blood pressure med. I had to call 3 pharmacies before one knew what NTI meant. 😅
Suzanne Johnston December 19, 2025
Is the real issue not the law, but the erosion of trust? We've been conditioned to believe that cost-efficiency equals safety. But the human body doesn't run on spreadsheets. The pharmacist's role as a gatekeeper of well-being is being replaced by a transactional algorithm.
precious amzy December 19, 2025
How quaint. You treat this as a policy problem. It's a metaphysical one. The assumption that 'equivalence' can be quantified by the FDA is a secular religion. The soul of medicine is lost when we reduce healing to chemical equivalence.
Maria Elisha December 20, 2025
My pharmacy just switched my antidepressant without saying anything. I felt like a zombie for two weeks. Then I noticed the pill was a different shape. I went back and they were like 'oh yeah, that's normal.' No. It's not.
Andrea Beilstein December 22, 2025
In my village in Oaxaca, the local healer knows every herb and its subtle variation. Here, we trust a machine to decide if a pill is 'equivalent.' We've lost the art of listening to the body. The real crisis isn't the law - it's the silence between patient and provider.
Shubham Mathur December 24, 2025
Guys let's be real. If you're on warfarin or levothyroxine you should never take a generic unless your doctor says so and you've been on the same brand for a year. I work in pharma and I've seen the lab reports. The dissolution profiles vary. It's not just placebo. It's physics. Stop being lazy and ask for the name
Angela R. Cartes December 26, 2025
So what? You got a different pill. Big deal. You're not dying. You're just saving $20. Chill. The system works. Your anxiety is the real NTI drug here 😘
William Umstattd December 27, 2025
That’s exactly what happened to my cousin in Texas. She had a seizure because they switched her generic. The pharmacist didn’t even know the drug was on the NTI list. Now her family’s suing the state.