When a pharmacist hands you a generic pill instead of the brand-name version, how do you know it’s truly the same? It’s not just about cost - it’s about safety, effectiveness, and trust. The FDA Orange Book is the official source that answers that question. It doesn’t just list drugs. It tells you which generics can be swapped in without risking your health.
What the FDA Orange Book Actually Does
The FDA Orange Book, officially called Approved Drug Products with Therapeutic Equivalence Evaluations, is the only government-backed database that rates whether a generic drug is a true substitute for its brand-name counterpart. It’s not a marketing tool. It’s a scientific evaluation.
Every drug in the Orange Book has been reviewed by the FDA for three key things: does it contain the same active ingredient? Is it absorbed the same way in your body? Is it made under the same strict quality standards? If yes, it gets an AB rating. That’s the green light for substitution.
Since 1984, the Orange Book has been the backbone of the U.S. generic drug system. Over 90% of prescriptions in the U.S. are filled with generics - and nearly all of them rely on this database to confirm they’re safe to swap. Without it, pharmacies couldn’t legally substitute drugs in most states.
Understanding Therapeutic Equivalence Codes
The Orange Book doesn’t just say “yes” or “no.” It uses a two-letter code system that tells you exactly how a drug compares.
- AB - This is what you want. It means the generic is therapeutically equivalent to the brand. Same active ingredient, same dosage, same absorption rate. It can be substituted without a doctor’s permission in most states.
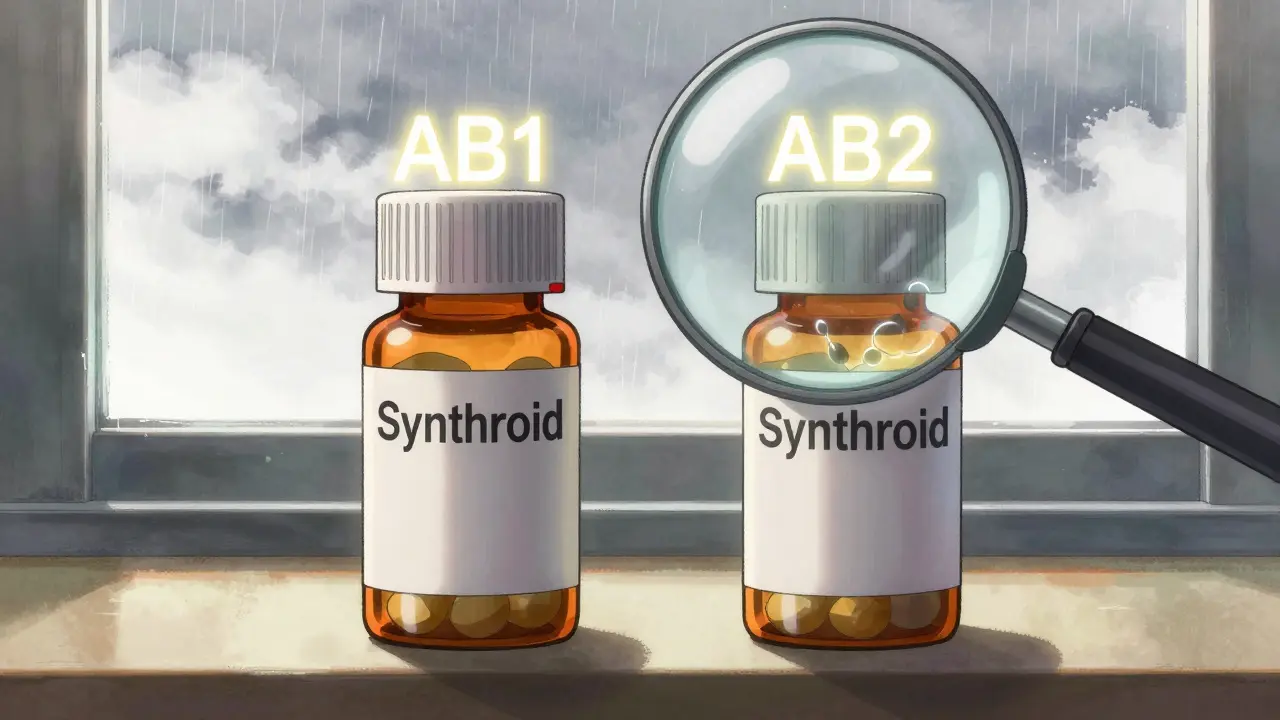
- AB1, AB2, AB3 - These mean the generic matches one of several possible brand-name versions (called Reference Listed Drugs). If your doctor prescribed a specific brand, you need to match the number. Mixing AB1 and AB2 isn’t always safe.
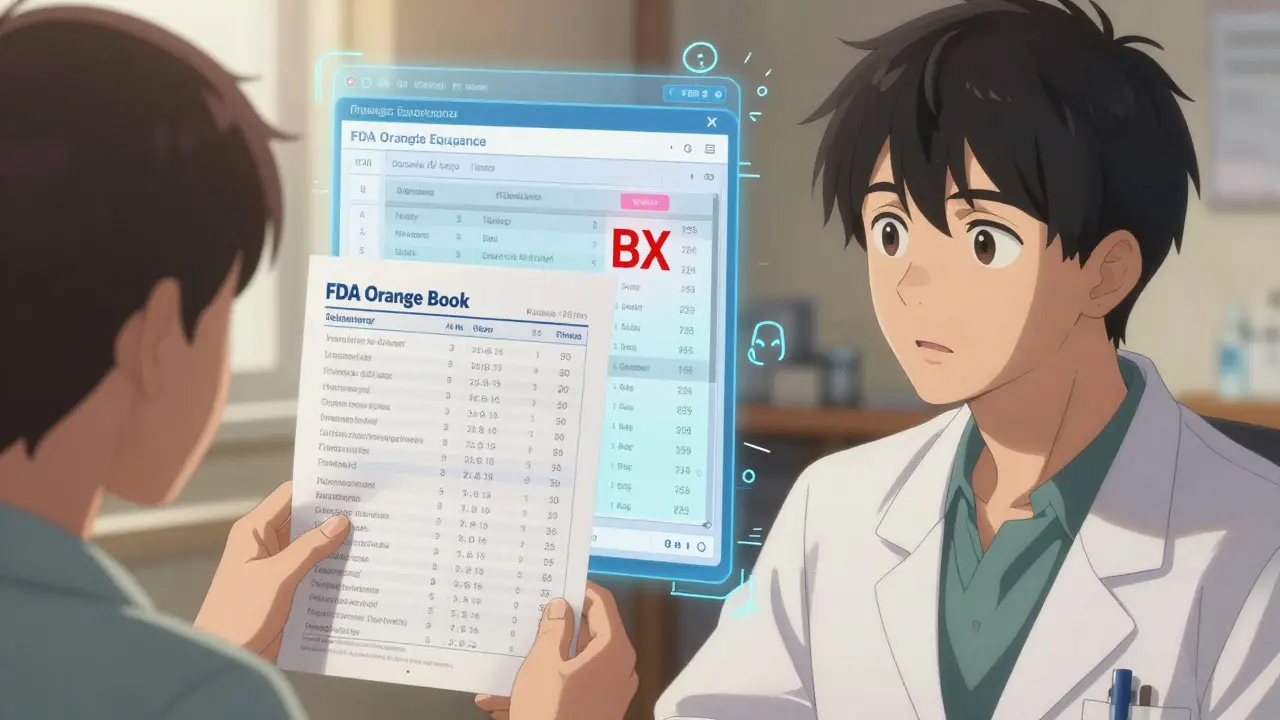
- B - This is a red flag. The FDA says this product isn’t reliably equivalent. Maybe the absorption is too unpredictable. Don’t substitute these unless your doctor specifically says so.
- BX - A subset of B. These are drugs with known bioequivalence issues. Think thyroid meds, seizure drugs, blood thinners. Even if labeled “generic,” they’re not interchangeable without close monitoring.
Here’s the catch: the code doesn’t change just because the manufacturer changes. If two different companies make levothyroxine and both get an AB rating, they’re still not automatically interchangeable with each other if they’re rated AB1 and AB2. That’s because they were tested against different brand-name originals.
How to Search the Electronic Orange Book
The old printed Orange Book is gone. Everything’s online now at accessdata.fda.gov/scripts/cder/ob/. Here’s how to use it correctly:
- Start with the brand name. Type in something like “Lipitor” or “Synthroid.” Don’t use the generic name yet - you’re trying to find the reference drug.
- Look for the “Reference Listed Drug” (RLD) column. The brand-name drug will say “Yes.” The generics will say “No.”
- Check the “Therapeutic Equivalence Code” column. Find the AB rating. If it’s missing, the product isn’t rated - that means it’s either discontinued or an OTC drug (which aren’t evaluated).
- Click on the generic product. It will show you which RLD it was tested against. Match the AB number if multiple exist.
- Double-check the dosage form. A 10mg tablet might be AB-rated, but the 20mg capsule might not be. They’re different products.
Pro tip: Use the “Ingredient Search” if you’re unsure of the brand name. Type in “levothyroxine” and sort by dosage form. You’ll see every approved version - brand and generic - with their codes side by side.
What the Orange Book Doesn’t Tell You
The Orange Book is authoritative - but it’s not complete.
First, it doesn’t mention state laws. In California, New York, and several other states, even AB-rated thyroid or epilepsy drugs require a doctor’s note before substitution. The FDA doesn’t track those rules. You have to check your state’s pharmacy board website separately.
Second, it doesn’t warn about delivery systems. Two inhalers might have the same active ingredient and AB rating, but if one uses a different propellant or spacer design, it might not work the same for a patient. The Orange Book doesn’t measure that.
Third, it doesn’t update instantly. The database is updated daily, but if a new generic is approved on Monday, it might not show up until Tuesday afternoon. Don’t rely on third-party apps like Drugs.com or Medscape - they can be 24 to 72 hours behind.
Common Mistakes Pharmacists and Patients Make
Even professionals get tripped up.
- Confusing patent expiration with market exclusivity. Just because a patent expired doesn’t mean a generic is approved. The Orange Book shows both, but they’re different dates.
- Assuming all generics with the same name are equal. Two “metformin” tablets might both be AB-rated, but if one is AB1 and the other is AB2, they’re not interchangeable.
- Ignoring discontinued products. If a drug isn’t in the main list, check the “Discontinued Drug Product List.” It’s easy to miss, but it’s there for a reason - those drugs are no longer made or approved.
- Using OTC drugs as a reference. The Orange Book doesn’t rate over-the-counter medicines like ibuprofen or antacids. Don’t try to apply AB ratings to them.
A 2023 survey of 1,250 U.S. pharmacists found that 42% struggled with interpreting TE codes for complex products like inhalers, topical creams, or injectables. Even experienced staff sometimes mix up AB1 and AB2.
When to Trust the Orange Book - and When to Double-Check
The Orange Book is your starting point, not your final answer.
Use it when:
- You’re verifying a generic before dispensing
- You’re comparing prices and need to confirm substitution is allowed
- You’re reviewing a formulary list for a health plan
Double-check with your doctor or pharmacist when:
- The drug has a narrow therapeutic index (thyroid, epilepsy, warfarin, digoxin)
- You’ve had a bad reaction to a previous generic
- State law requires physician approval for substitution
- The product is an inhaler, patch, or liquid suspension
For high-risk drugs, the FDA itself says: “Therapeutic equivalence does not guarantee identical performance in all patients.”
What’s Next for the Orange Book
The FDA is modernizing the Orange Book. By 2024, it will integrate with the Purple Book (for biologics like Humira and Enbrel). That means you’ll soon be able to check biosimilar substitutions in one place.
They’re also moving to machine-readable data formats. Soon, pharmacy systems and EHRs will pull Orange Book data automatically - no more manual searches.
But for now, the best tool is still the official Electronic Orange Book. It’s free. It’s updated daily. And it’s the only source the FDA legally recognizes.
Quick Reference: How to Verify in 5 Steps
- Go to accessdata.fda.gov/scripts/cder/ob/
- Search by brand name or active ingredient
- Find the Reference Listed Drug (RLD = Yes)
- Look for the Therapeutic Equivalence Code - only AB-rated products are interchangeable
- Check the AB number (AB1, AB2) if multiple RLDs exist - match the exact one
Save this process. Print it. Keep it near your workstation. You’ll use it more than you think.
Is the FDA Orange Book free to use?
Yes. The Electronic Orange Book is publicly available at no cost on the FDA’s website. There are no subscriptions, logins, or fees. Third-party websites may charge for access, but they’re just repackaging the same free data - often with delays.
Can I substitute any AB-rated generic for a brand-name drug?
Legally, yes - but only if your state allows it. Most states permit substitution for AB-rated drugs, but some require a doctor’s note for drugs with narrow therapeutic windows, like levothyroxine or warfarin. Always check your state’s pharmacy board rules.
What does AB1 vs AB2 mean?
It means the generic was tested against different brand-name versions (Reference Listed Drugs). For example, one generic might be tested against Synthroid (AB1), another against Levoxyl (AB2). Even though both are AB-rated, they’re not interchangeable with each other unless they’re rated against the same RLD.
Are over-the-counter (OTC) drugs listed in the Orange Book?
No. The Orange Book only covers prescription drugs. OTC medications like ibuprofen, loratadine, or omeprazole are not evaluated for therapeutic equivalence. That’s why you won’t see AB ratings on store-brand pain relievers.
Why do some generics have no TE code?
If a product has no TE code, it’s either discontinued, not approved for substitution, or an OTC drug. Check the Discontinued Drug Product List on the FDA site. If it’s not there and has no code, it’s not eligible for generic substitution.
Can I trust Drugs.com or Medscape instead of the FDA Orange Book?
No. While these sites are helpful for quick lookups, they lag behind the FDA’s official database by up to 72 hours. In urgent cases - like switching a patient’s blood thinner - you must use the FDA’s site. It’s the only legally recognized source.
Next Steps
If you’re a pharmacist, spend 15 minutes today searching for three generics you commonly dispense. Compare their TE codes. Notice how many have AB1 vs AB2. That’s your training.
If you’re a patient, ask your pharmacist: “Is this generic rated AB? And which RLD is it matched to?” If they hesitate, ask to see the FDA Orange Book. You have the right to know.
Generic drugs save billions. But they only work if they’re truly equivalent. The FDA Orange Book is how we make sure they are.






Erika Putri Aldana December 20, 2025
lol so now i gotta check the orange book before i take my metformin? 😒 i just want my cheap pills and not a phd in pharmacology.
Grace Rehman December 22, 2025
the orange book is basically the FDA’s way of saying 'trust us, we did the math'... but then you find out AB1 and AB2 aren’t interchangeable and suddenly you’re questioning everything you thought you knew about medicine. 🤔
Meina Taiwo December 23, 2025
AB rating = safe to swap. BX = don't touch. Simple. Use the FDA site. No apps.
Dan Adkins December 25, 2025
It is imperative to note that the therapeutic equivalence codes are not merely administrative designations, but rather evidence-based determinations grounded in bioequivalence studies conducted under controlled clinical conditions. Failure to adhere to these distinctions constitutes a potential breach of pharmaceutical standards and may result in clinically significant deviations in plasma concentration profiles.
Adrian Thompson December 26, 2025
they don't want you to know this. the orange book is a lie. big pharma owns the FDA. they let in bad generics so you get sick and keep buying more. i checked three drugs - all AB rated - but my cousin's blood pressure spiked after switching. coincidence? i think not.
Southern NH Pagan Pride December 27, 2025
the orange book is a distraction. the real issue is the cdc and who are colluding with the pharmas to push generics so they can track your biometrics through your pill bottles. you think they care if it's ab1 or ab2? they just want your data. and your money.
Jackie Be December 27, 2025
OMG I JUST REALIZED I’VE BEEN TAKING AB2 WHEN I NEEDED AB1 FOR MY THYROID AND I DIDN’T EVEN KNOW IT 😭😭😭 I’M GOING TO THE PHARMACY RIGHT NOW AND I’M TAKING THE ORANGE BOOK WITH ME
Jerry Peterson December 29, 2025
i used to think generics were all the same until i got a bad reaction to one. now i always check the orange book. it’s not hard, and it’s free. just type in the brand name, look for AB, and match the number. saved my life.
John Hay December 29, 2025
i work at a pharmacy and 70% of patients don’t know what the orange book is. we show them. they’re shocked. it’s not complicated. just open the site. look at the code. done. no need to panic.
Stacey Smith December 30, 2025
if you're not using the official FDA site you're playing russian roulette with your meds. period. stop trusting third party apps. they're slow. they're wrong. the orange book is the law.
Peggy Adams December 31, 2025
the orange book is just another government scam. i got my generic thyroid med and it made me feel like a zombie. the brand worked fine. now i pay extra because i don't trust the system. and honestly? neither should you.