Every year, Americans fill over 3.9 billion prescriptions for generic medicines. That’s nine out of every ten prescriptions written. And yet, these pills and capsules account for just 12% of total drug spending. Meanwhile, brand-name drugs-filled in fewer than 435 million prescriptions-consume 88% of the $700 billion spent annually on prescription drugs. This isn’t a glitch. It’s the system working as designed. And it’s saving the U.S. healthcare system over $467 billion every year.
How Generics Cut Costs Without Cutting Corners
Generic drugs aren’t cheap because they’re low quality. They’re cheap because they don’t need to repeat the billion-dollar clinical trials that brand-name drugs do. Once a patent expires, other manufacturers can prove their version is bioequivalent-meaning it delivers the same active ingredient, in the same dose, the same way, with the same effect. The FDA requires this. No exceptions. Take metformin, the most prescribed diabetes drug in the U.S. The brand version, Glucophage, used to cost over $200 a month. Today, a 90-day supply of generic metformin costs about $10 at most pharmacies. That’s a 95% drop. And it’s not just diabetes. The top 10 generic drugs in 2024 treated conditions like high blood pressure, high cholesterol, depression, and asthma. Together, they saved patients and insurers $127 billion in just one year. The savings aren’t just theoretical. In 2024, Medicare saved $142 billion and Medicaid saved $62.1 billion because patients were prescribed generics instead of brands. For millions of seniors on fixed incomes, that’s the difference between taking their medication or skipping doses.Biosimilars: The Next Wave of Savings
While generics copied small-molecule drugs, biosimilars are doing the same for complex biologic drugs-medications made from living cells, like insulin, rheumatoid arthritis treatments, and cancer therapies. These used to cost $10,000 to $20,000 a year. The first biosimilar entered the U.S. market in 2015. Since then, they’ve saved $56.2 billion. In 2024 alone, biosimilars saved $20.2 billion. One example: adalimumab (Humira), once the most expensive drug in the U.S., now has over a dozen biosimilars on the market. Prices dropped by 70% within two years. A patient who paid $7,000 a year for Humira now pays under $2,000 with a biosimilar. That’s $5,000 saved per person, per year. The catch? Biosimilars are harder to get approved. They require more testing than traditional generics. But as more get approved-17 in 2024 alone-and as more gain “interchangeable” status (meaning pharmacists can swap them without doctor approval), savings will accelerate.Why the U.S. Saves More Than Any Other Country
The U.S. fills 90% of prescriptions with generics. In Germany? About 70%. In the UK? Around 80%. Why? Because the U.S. system was built to encourage competition. The 1984 Hatch-Waxman Act created a clear path for generic manufacturers to enter the market. It gave the first company to file a generic version a 180-day exclusivity period-enough to make a big profit and incentivize others to follow. That’s why the U.S. has over 1,145 new generic approvals each year, more than any other country. But here’s the twist: even though the U.S. uses generics more, it still pays more for them than other countries. A 30-day supply of lisinopril (a blood pressure drug) costs $4 in Canada and $12 in the U.S. Why? Because the system is broken in places.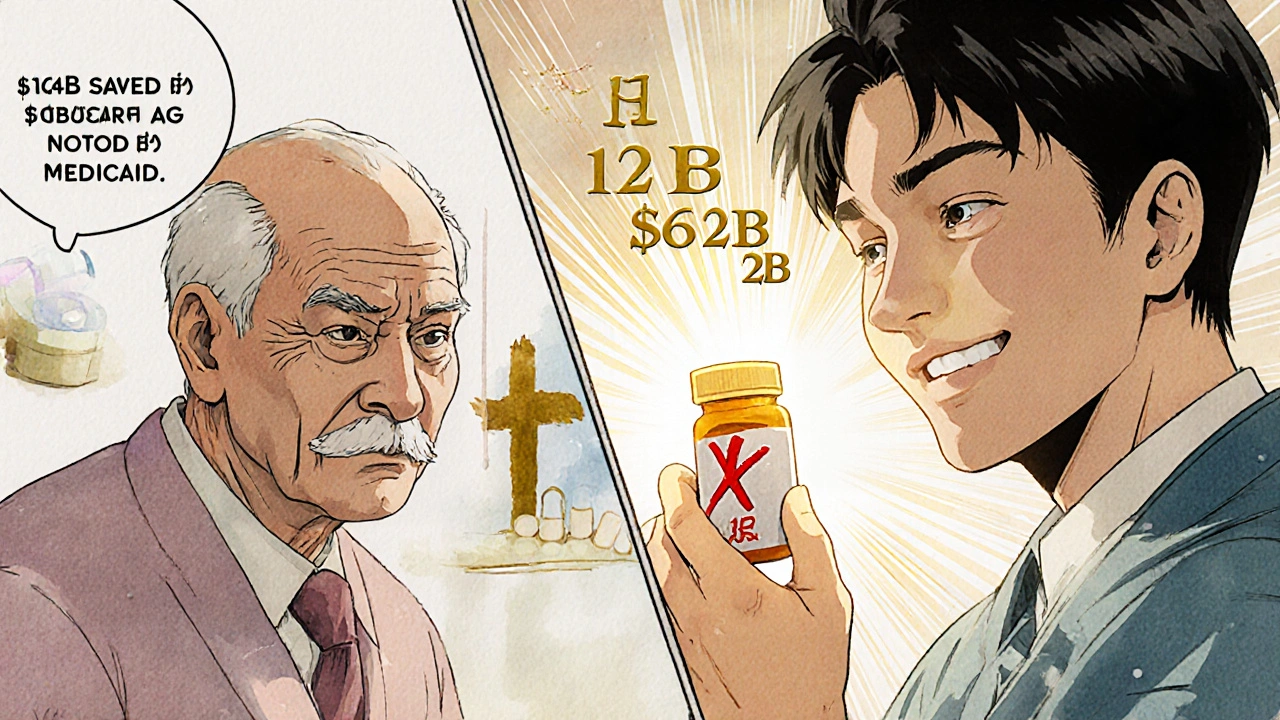
The Hidden Costs: How Big Pharma Blocks Savings
For every dollar saved by generics, there’s a company fighting to protect its monopoly. Here’s how:- Patent thickets: Brand companies file dozens of secondary patents on minor changes-like a new coating or tablet shape-to delay generics. A 2024 JAMA study found just four drugs used this tactic to block competition, costing the system over $3.5 billion in two years.
- Pay-for-delay: Sometimes, brand companies pay generic makers to stay off the market. In 2023, these deals cost federal programs $3 billion and drove up prices by $12 billion nationwide.
- Product hopping: A company switches from an old drug to a new version with a fresh patent, then pressures doctors and insurers to stop prescribing the old, generic-friendly version. This tactic cost $1.1 billion in federal spending over ten years, according to the Congressional Budget Office.
- Citizen petitions: Brand companies file fake safety complaints with the FDA to delay generic approval. In 2023, there were 118 of these-up from 37 in 2018.
Who’s Really Saving Money?
The biggest winners? Patients. Insurers. Medicare. Medicaid. A 2023 survey of 500 patients found that 89% who switched to generics were happy with both cost and effectiveness. On average, they saved $147 per medication each month. For someone on three generics, that’s over $1,700 a year. But not everyone wins. Pharmacy Benefit Managers (PBMs)-the middlemen between insurers, pharmacies, and drug makers-sometimes push brand drugs because they get bigger rebates. A 2024 report from Express Scripts showed that their formulary design saved $18.3 billion by favoring generics. But other PBMs don’t. And some Medicare Part D plans still list brand drugs as preferred, even when generics are available. State laws help. California mandates pharmacists substitute generics unless the doctor says no. Result? 98% generic use. Texas lets pharmacists substitute, but doesn’t require it. Result? 87% use. The difference? Over $1 billion in annual savings.
The Future: More Generics, But Not Without a Fight
The pipeline is full. Over $24 billion in brand drugs are set to lose patent protection by 2025. That includes drugs for heart disease, mental health, and autoimmune conditions. If nothing blocks them, these could save $10-$15 billion in just two years. Congress is trying. The Affordable Prescriptions for Patients Act, passed by the Senate HELP Committee in June 2024, would crack down on patent abuse and pay-for-delay deals. The Congressional Budget Office estimates it would save $7.2 billion a year. But threats remain. Drug shortages hit 287 generic medications in December 2024. Many are made overseas, and supply chain issues-especially in India and China-can cause delays. Also, the market is consolidating. The top 10 generic manufacturers now control 63% of the market, up from 51% in 2015. Less competition means less pressure to lower prices. Still, the math is clear. Generics and biosimilars have saved $3.4 trillion since 2015. By 2034, that number could hit $5.1 trillion if the system works as intended.What You Can Do
If you’re on a prescription:- Ask your doctor: “Is there a generic version?”
- Ask your pharmacist: “Can you substitute?”
- Check your Medicare Part D plan’s formulary. If the brand is preferred over a generic, call and ask why.
- Use GoodRx or SingleCare to compare cash prices. Sometimes, the cash price for a generic is lower than your copay.
Are generic drugs as safe and effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also meet the same strict manufacturing standards. Over 90% of generics are rated “A” by the FDA, meaning they’re therapeutically equivalent. A 2025 Drugs.com analysis of over 15,000 reviews found that 87% of users rated cost as excellent or good, and 63% rated effectiveness the same.
Why do some people say generics don’t work as well?
Some patients notice differences in inactive ingredients-like fillers or dyes-which can affect how a pill dissolves. For most drugs, this doesn’t matter. But for narrow-therapeutic-index drugs like warfarin or levothyroxine, even small changes can have effects. In those cases, sticking with one manufacturer is advised. Most patients, however, experience no difference at all.
Do biosimilars save as much as generics?
Not yet, but they’re catching up. Generics cut prices by 80-95%. Biosimilars typically cut prices by 30-70% because they’re harder to produce. Still, they’ve saved $56.2 billion since 2015. As more gain interchangeable status and competition grows, savings will increase. For high-cost drugs like Humira, biosimilars have already saved patients thousands per year.
Why are generic drug prices still high in the U.S. compared to other countries?
The U.S. doesn’t negotiate drug prices like other countries. In Canada, the UK, or Germany, governments set price caps. In the U.S., prices are set by market forces-PBMs, insurers, and pharmacies. Even with competition, middlemen sometimes keep prices high. A 2022 study found U.S. consumers pay 2-3 times more for the same generic than in other developed nations.
Can I trust generics from other countries?
Only if they’re approved by the FDA. Many generic drugs sold in the U.S. are made in India or China, but they must meet U.S. standards. Drugs bought online from foreign pharmacies aren’t regulated and may be counterfeit or ineffective. Always fill prescriptions at licensed U.S. pharmacies.







Dana Dolan November 20, 2025
Just got my metformin refill for $8 at Walmart. I used to pay $180 for the brand. No difference in how I feel. Why are we still paying more for the same thing? The system is broken, but at least I win.
Also, my pharmacist automatically swaps generics now. No questions asked. Love it.
Steve and Charlie Maidment November 22, 2025
You know what’s wild? The fact that these same companies that make generics are the same ones that make the brand-name drugs. It’s all the same factory, same chemists, same quality control. They just slap a different label on it and charge you 20x more. The whole thing is a pyramid scheme dressed in white coats.
And don’t even get me started on how PBMs get kickbacks from brand manufacturers to push expensive drugs even when generics exist. It’s not about saving money-it’s about who gets the cut.
Michael Petesch November 22, 2025
The Hatch-Waxman Act of 1984 remains one of the most consequential pieces of pharmaceutical legislation in U.S. history. It successfully balanced innovation incentives with market competition, creating a legal pathway for generic entry without compromising safety standards.
What is less discussed is how the FDA’s Abbreviated New Drug Application (ANDA) process-while rigorous-has been systematically exploited by brand manufacturers through evergreening tactics. The data on patent thickets and product hopping is not anecdotal; it is empirically documented in Congressional Research Service reports dating back to 2017.
Moreover, the rise of biosimilars introduces a new regulatory frontier. Unlike small-molecule generics, biosimilars require analytical, preclinical, and clinical data to demonstrate similarity. This complexity delays market entry, even as demand increases. The FDA’s recent guidance on interchangeability status may accelerate adoption, but only if reimbursement policies align.
Ellen Calnan November 22, 2025
I used to be one of those people who thought generics were ‘the cheap stuff’-until my mom got diagnosed with hypertension and we were looking at $400/month for her meds.
Switched to lisinopril generic. $4. Same pill. Same results. She cried when she saw the receipt.
And then I found out that the same company that makes her $4 generic also makes the $400 brand version. The only difference? The logo on the bottle.
It’s not just about money. It’s about dignity. People shouldn’t have to choose between food and their heartbeat. Generics aren’t a compromise-they’re justice.
And if you’re still skeptical? Go ask someone on Social Security who’s been taking a generic for 10 years. They’ll tell you the truth: they feel fine. They’re alive. And they’re grateful.
Don’t let corporate spin make you doubt what’s right in front of you.
Also, GoodRx is a miracle app. Use it.
Richard Risemberg November 24, 2025
Let me tell you something that’ll blow your mind: the FDA doesn’t care if your pill is blue or green or shaped like a star. They care about the active ingredient, the dissolution rate, and the bioavailability. That’s it.
So when someone says, ‘My generic doesn’t work like the brand,’ what they’re really saying is, ‘I’m used to the branding, the packaging, the placebo effect of paying more.’
But here’s the kicker-sometimes, the inactive ingredients DO matter. For drugs like levothyroxine or warfarin, consistency matters. That’s why some docs still prescribe the same brand. Not because it’s better-but because switching manufacturers could mean a tiny shift in absorption.
That’s why pharmacists should be allowed to stick with one generic manufacturer unless you ask for a change. It’s not about brand loyalty-it’s about stability.
And let’s not forget: the real villains aren’t the generic makers. They’re the ones who file 50 patents on a pill’s coating just to delay a $3 version hitting shelves.
Also, biosimilars? They’re the future. Insulin that costs $20 instead of $300? Yes please. Let’s make that the norm.
Andrew Montandon November 24, 2025
Wait, so the FDA says generics are bioequivalent, and yet people still complain? That’s like saying a Ford F-150 isn’t as good as a Cadillac Escalade because it doesn’t have leather seats.
They both get you from point A to point B. One just costs 3x more and has a fancy name.
And don’t even get me started on PBMs-they’re the real drug cartel. They negotiate rebates with brand manufacturers, then pass the savings to insurers… but not to you. So you pay more out-of-pocket even when a cheaper generic exists.
California’s law forcing substitution? Genius. Texas? Still playing catch-up.
Also, if your doctor doesn’t know the difference between a brand and generic, ask for a pharmacist consultation. They’re the real heroes here.
Sam Reicks November 24, 2025
Generics are a government scam to control us
Big Pharma and the FDA are in cahoots to make you think you're saving money but really they're just swapping one monopoly for another
Did you know the same CEOs own both the brand and generic companies?
And why do you think they let generics in at all? So they can charge you less... but only after they've milked you for 20 years
Also, all the data is fake. The FDA is bought. The studies? Sponsored by the same corporations that make the pills
And why is India making most of them? Because they don't care if you die
Wake up
They want you dependent on pills
And they want you to think you're smart for using generics
It's all a trap
Chuck Coffer November 25, 2025
Wow. Another article celebrating how much we’re being ‘saved’ by the system that literally invented price gouging.
Let’s not pretend generics are some noble victory. They’re what happens when the monopoly finally cracks under public pressure.
And yes, they’re cheaper-but only because the brand-name version was priced like a luxury watch. That’s not innovation. That’s extortion.
Also, ‘90% of prescriptions are generic’? Cute. But the top 1% of drugs still take up 80% of spending. So who’s really benefiting?
And don’t even get me started on the fact that the same people who profit from brand drugs are now profiting from biosimilars. It’s the same game, just with a new name.
Generics didn’t save us. They just made the scam slightly less obvious.
Marjorie Antoniou November 25, 2025
I work in a community clinic. I see patients who skip doses because they can’t afford their meds.
One woman told me she was taking half her insulin pill because the brand cost $500 a month. She was 72. Had no family nearby.
We switched her to the generic. $12 a month. She cried. Not because she was sad-because she could finally sleep at night.
That’s not a statistic. That’s real life.
And yes, sometimes the pill looks different. Sometimes the taste is off. But the effect? The same.
Let’s stop pretending this is about quality. It’s about access.
And if you’re still skeptical? Talk to someone who’s been on a generic for five years. They’ll tell you the truth: they’re still alive. And they’re grateful.
Andrew Baggley November 27, 2025
Let’s be real-this isn’t about drugs. It’s about dignity.
Imagine being told your life depends on a pill… and then being told it costs more than your rent.
Generics don’t just lower prices. They lower the shame.
I used to feel guilty for taking ‘cheap’ meds. Now I feel proud. I’m not buying a brand-I’m buying survival.
And if you think generics are ‘lesser,’ go ask the veteran who’s been on generic warfarin for 12 years. Or the kid with asthma who breathes easier because his inhaler costs $5 instead of $300.
This isn’t a compromise. It’s a revolution.
And we’re winning.
Keep fighting for the next one.
Also-GoodRx. Always check it.
Frank Dahlmeyer November 29, 2025
It’s fascinating how the U.S. system, despite its flaws, has managed to create a massive generic market through competition rather than centralized price controls. Other countries cap prices, but they also stifle innovation and delay access. Here, we let the market do the work-except when it’s rigged by patent thickets and pay-for-delay deals.
The fact that over 1,145 new generic approvals happen annually in the U.S. is a testament to the power of legal incentives. The Hatch-Waxman Act didn’t just allow generics-it created an entire industry around them.
But the real story isn’t just the savings-it’s the behavioral shift. Patients now expect generics. Pharmacists routinely substitute. Doctors routinely prescribe them. That cultural shift is harder to reverse than any law.
And biosimilars? They’re the next frontier. The fact that Humira’s price dropped 70% in two years after biosimilars entered? That’s market power in action.
The only thing holding us back now is the lobbying power of companies who profit from confusion.
Let’s not let them win.
Codie Wagers November 30, 2025
It’s ironic that we celebrate generics as a triumph of capitalism, when in reality, capitalism-unregulated-is precisely what created this crisis.
The brand-name drugs were priced at astronomical levels because the system allowed it. The generics didn’t fix the system-they exploited a loophole in it.
And now, the same corporations that once held monopolies are the ones manufacturing the generics-so they still profit, just under a different label.
The real problem? We’ve normalized exploitation. We’re thrilled that a pill costs $10 instead of $200, but we don’t ask why it was $200 in the first place.
Generics are a Band-Aid on a hemorrhage.
And the fact that we’re proud of this? That’s the tragedy.
We should be outraged that life-saving medicine was ever priced like a luxury good.
But no. We’re just grateful for crumbs.
Paige Lund December 2, 2025
So… we’re supposed to be impressed that we’re not getting ripped off as badly as before? Cool. I guess that’s something.
Meanwhile, my insurance still won’t cover the generic unless I jump through 17 hoops.
Thanks, system.
Ellen Calnan December 3, 2025
And yet-people still think generics are ‘inferior’ because they’re cheaper. It’s like saying a Toyota is worse than a BMW because it doesn’t have heated seats.
But here’s the thing: the Toyota gets you to work. The BMW gets you a parking ticket.
And the people who need the Toyota? They’re the ones who can’t afford the ticket.
So yeah. Let’s keep fighting for the $4 pills. Not because they’re ‘good enough’-but because they’re everything.