When you walk into a pharmacy and see two identical pills side by side-one labeled with a big brand name, the other with a simple generic label-why does the generic cost so much less? If they’re the same drug, why isn’t the price the same? The answer isn’t just about generic drugs. It’s about authorized generics.
What Exactly Is an Authorized Generic?
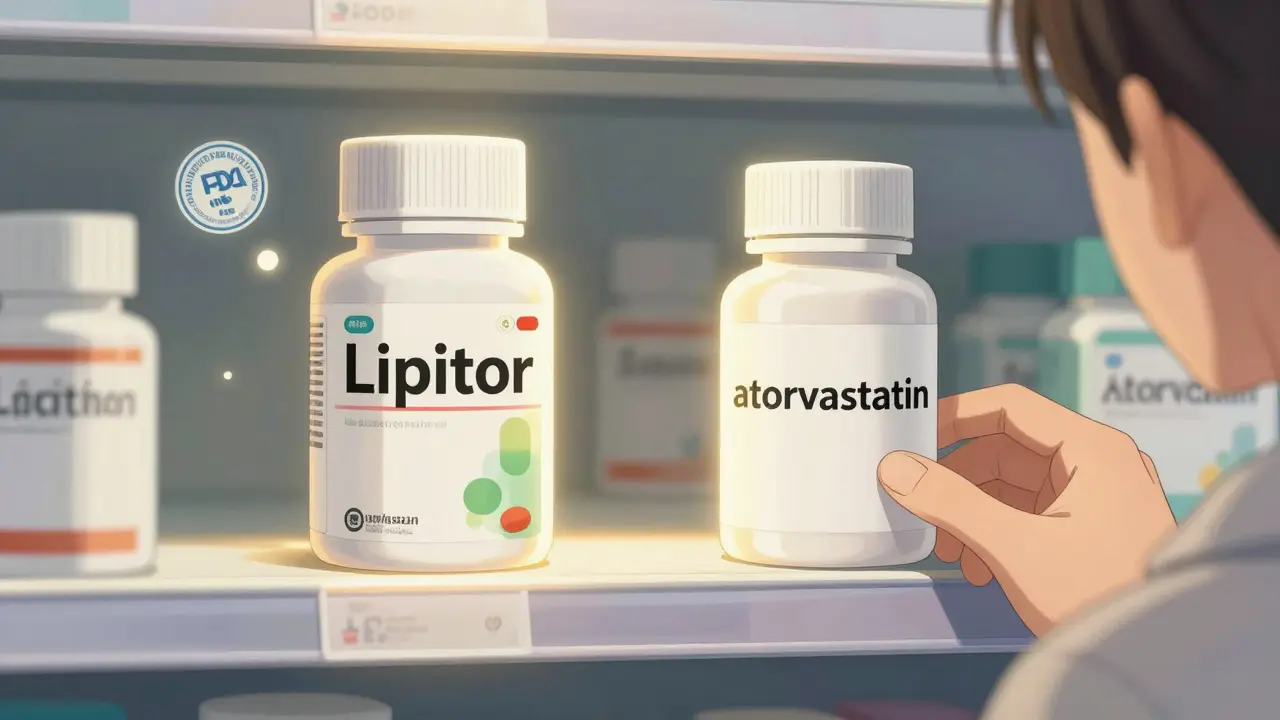
An authorized generic is the exact same medicine as the brand-name drug. Same active ingredient. Same capsule, tablet, or injection. Same factory. Same quality control. The only difference? The label. It doesn’t say "Lipitor" or "Viagra." It says something like "atorvastatin" or "sildenafil." But here’s the twist: it’s made by the original brand company-or licensed to another company with the brand company’s permission. The U.S. Food and Drug Administration (FDA) keeps a public list of these. As of October 2023, there were 137 active authorized generics on the list. These aren’t knockoffs. They’re the real thing, just sold under a different name. That’s why they’re called "authorized." The brand company approves it. The FDA monitors it. And because they use the same New Drug Application (NDA) as the brand, they skip the usual generic approval process. No extra testing. No delays.Why Do Authorized Generics Cost Less?
The short answer: no marketing. No patents. No brand loyalty. But there’s more to it. Brand-name drugs come with a huge price tag because the company spent years and billions developing the drug, running clinical trials, and advertising. Once the patent expires, other companies can make copies. But here’s where it gets interesting. The original brand company doesn’t just sit back and watch their sales vanish. Many launch their own authorized generic at the same time as the first generic competitor. Why? To stay in the game. When a brand company releases an authorized generic, they’re not giving up-they’re competing. They know that if they don’t, the first generic maker will charge a high price during their 180-day exclusivity window. That’s a rule from the 1984 Hatch-Waxman Act. It gives the first generic company a head start with no competition. But if the brand company drops its own version at the same time, that first generic can’t charge top dollar anymore. They have to slash prices to stay competitive. The result? Retail prices for the drug drop 4% to 8% compared to when no authorized generic is present. For pharmacies buying in bulk, the discount is even bigger-up to 18% lower than if the brand had no competition at all. That’s not just savings for insurers. That’s savings for you at the counter.Authorized Generics vs. Regular Generics
Not all generics are the same. Here’s how they differ:- Regular generics: Made by a different company after the patent expires. They go through a separate FDA approval process called an Abbreviated New Drug Application (ANDA). They’re cheaper than the brand, but not always as cheap as an authorized generic.
- Authorized generics: Made by the brand company or under its license. Same factory, same formula, same packaging (except the name). No ANDA needed. They enter the market on day one of generic competition and immediately push prices down.

How Authorized Generics Change the Market
Before authorized generics, the first generic company had a free pass. They could set a high price, knowing no one else could compete for six months. That meant patients paid more, insurers paid more, and Medicare paid more. Now? The moment a brand’s patent expires, you might see three versions on the shelf:- The original brand-name drug
- An authorized generic
- A regular generic from another company
Real Examples: When Authorized Generics Made a Difference
In 2016, Mylan faced backlash for raising the price of the EpiPen from $100 to $600. Then they released an authorized generic for $300. That wasn’t charity. It was damage control. But it also gave people a real, affordable option. Gilead did something similar with its hepatitis C drugs Harvoni and Epclusa. Before their patents expired, they launched authorized generics to keep market share as cheaper alternatives loomed. They didn’t wait for competition to hit-they started it themselves. And it worked. In both cases, patients got lower prices. Pharmacies got better margins. And the brand companies still made money-just not as much as they hoped.Why Your Pharmacy Might Not Tell You About Them
Here’s the catch: authorized generics aren’t always easy to find. Pharmacy Benefit Managers (PBMs)-the middlemen between insurers and pharmacies-decide which drugs get placed on which tiers. Sometimes, they put the brand-name drug and the authorized generic on the same tier. That means your copay is the same for both. You might not even know you’re paying the same price for the exact same drug. But if the PBM puts the authorized generic on a lower tier, you save. A 2022 analysis of 1.2 million Medicare Part D patients showed that when AGs were placed on the same tier as regular generics, medication adherence went up by 8.2 percentage points. People took their meds more consistently because they could afford them. The problem? PBMs don’t always make that choice. Some still favor the brand. Others hide the authorized generic behind complex pricing rules called Maximum Allowable Cost (MAC) lists. These lists set the max a plan will pay for a drug. But they’re often opaque. Thirty-two states now require PBMs to disclose how they set these prices. Still, it’s not universal.
Are Authorized Generics Safe?
Absolutely. The FDA requires them to meet the same standards as the brand. Same manufacturing facility. Same quality checks. Same batch testing. There’s no difference in safety or effectiveness. Dr. Aaron Kesselheim from Harvard Medical School says authorized generics "compel all market participants to adjust their pricing strategies from day one." That’s good for patients. It stops the "monopoly pricing" that happens when only one generic is allowed to compete. Some critics worry that brand companies use authorized generics as a way to delay real competition. The Federal Trade Commission has looked into this. In some cases, brand companies have paid generic makers to delay launching their own version-and then launched their own authorized generic instead. That’s called a "pay-for-delay" deal. It’s controversial. And the FTC is cracking down. But that doesn’t mean authorized generics themselves are bad. It means the system can be gamed. The drug itself? Still safe. Still effective. Still cheaper.What This Means for You
If you’re on a brand-name drug, ask your pharmacist: "Is there an authorized generic for this?" Don’t assume the generic you’re given is the cheapest option. Sometimes, the authorized version is cheaper than the regular generic. Check your formulary. If your insurance plan puts the brand and the authorized generic on the same tier, you’re not saving money. Ask your plan to move the authorized generic to a lower tier. And if you’re paying cash? Always compare the price of the brand, the regular generic, and the authorized generic. The difference can be $20, $50, even $100 a month. In 2023, the Inflation Reduction Act capped out-of-pocket drug costs for Medicare beneficiaries at $2,000 a year. Authorized generics play a big role in making that cap possible. They’re not just a corporate tactic. They’re a tool for affordability.The Bigger Picture
Authorized generics make up about 12% of the $60 billion U.S. generic drug market. That’s not huge-but their impact is huge. They force competition. They lower prices. They help people stick to their prescriptions. They’re not perfect. The system still has loopholes. PBMs still have too much power. But when it comes to getting the same medicine for less, authorized generics are one of the most reliable tools we have. The next time you pick up a prescription, don’t just look at the name on the bottle. Ask what’s underneath it. You might be paying more than you need to-for a drug that’s already been made, tested, and approved.Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are identical to their brand-name counterparts in dosage, strength, safety, quality, and how they work. They’re made in the same factory, using the same ingredients and processes. The only difference is the label and the price.
Why are authorized generics cheaper than brand-name drugs?
They don’t carry the marketing, advertising, and patent recovery costs that brand-name drugs do. Since they’re made by the original manufacturer or under its license, they skip the lengthy FDA approval process for generics. That cuts costs, and those savings get passed on.
Can I trust the quality of an authorized generic?
Yes. The FDA requires authorized generics to meet the same strict standards as brand-name drugs. They’re held to the same manufacturing and quality controls. There’s no difference in safety or effectiveness.
Why doesn’t my pharmacy always offer the authorized generic?
Pharmacy Benefit Managers (PBMs) decide which drugs go on which insurance tiers. Sometimes, they place the brand and the authorized generic on the same tier, so your copay is the same. Other times, they don’t promote the authorized version at all. Always ask your pharmacist or check your plan’s formulary.
Do authorized generics delay cheaper generic competition?
In some cases, yes. There have been instances where brand companies used authorized generics as part of "pay-for-delay" deals to block other generics from entering. The FTC is actively investigating these practices. But the authorized generic itself isn’t the problem-it’s how it’s used in some corporate strategies.
How can I find out if an authorized generic exists for my drug?
Ask your pharmacist directly. You can also check the FDA’s quarterly list of authorized generics online. Many pharmacy apps and insurance portals now show if an authorized generic is available and how it compares in price to the brand and other generics.






Emma ######### January 17, 2026
So basically, if I’m paying $120 for Lipitor, and there’s an authorized generic for $45, why does my pharmacy still push the brand? I’ve asked. They say it’s ‘on my tier.’ But my tier is the same for both. Smells like a PBM scam to me.
Andrew Qu January 18, 2026
Good breakdown. I work in a community pharmacy, and I can tell you-authorized generics are the quiet heroes of the Rx aisle. Patients don’t know they exist, and pharmacists aren’t always trained to push them. But when we do? Adherence spikes. People actually fill their scripts. That’s not corporate magic-that’s public health.
Nishant Sonuley January 19, 2026
Oh wow, so the same company that charged you $600 for an EpiPen is now selling you the exact same thing for $300 under a different name? How noble. 🤡 Let me guess-they just happened to ‘accidentally’ launch it right after the media storm? Classic. They don’t care about you. They care about market share. The FDA doesn’t regulate greed, only labels. And honestly? I’d rather pay $150 for the brand than fund their ‘authorized’ PR stunt. At least then I know who to hate.
Naomi Keyes January 20, 2026
According to the FDA’s 2023 Authorized Generic List (Revision 17.3, Section 4.2.1), 137 products are currently listed under the NDA-based pathway, which excludes ANDA-submitted generics. This distinction is critical, as authorized generics do not undergo bioequivalence testing under 21 CFR 314.94, but instead rely on the originator’s NDA data-thereby bypassing the 180-day exclusivity window entirely. This is a regulatory arbitrage mechanism, not a consumer benefit. The Hatch-Waxman Act was designed to incentivize generic competition, not enable monopolistic price suppression via corporate self-competition. The FTC should investigate whether this constitutes a form of reverse exclusion.
Jay Clarke January 21, 2026
They’re not saving you money. They’re saving Big Pharma’s reputation. You think they care about your insulin? No. They care about you not suing them. They launched authorized generics because the public was screaming. Not because they’re good people. They’re predators with a PR team.
Stacey Marsengill January 21, 2026
I used to take the brand for my blood pressure med. Then I switched to the authorized generic. My husband said I looked ‘less stressed.’ I cried. Not because it worked better-but because I finally stopped feeling like a sucker for paying $200 a month for a pill that costs $5 to make. I’m not mad. I’m just… tired. And grateful.
Ryan Otto January 22, 2026
Let me guess: the same corporations that lobbied against drug price negotiation are now the ones ‘saving’ you with ‘authorized generics’? This is stage-managed capitalism. The FDA is a puppet. The PBM is the puppeteer. And you? You’re the puppet’s paycheck. This isn’t transparency. It’s theater with a pill bottle.
Dayanara Villafuerte January 24, 2026
Yessssss!! 🙌 I’ve been telling my friends this for years!! Just last week I switched my antidepressant to the authorized generic-same pill, half the price, no side effects. My bank account did a happy dance 💃💸. Pharmacies should be REQUIRED to show you the AG option first. #PharmaIsARipoff
Max Sinclair January 26, 2026
Thank you for this. I’ve seen so many people confused between generics and authorized generics. This is one of those rare cases where the system actually works-for once. It’s not perfect, but asking your pharmacist is one of the easiest ways to save hundreds a year. Small actions, big impact.
Tyler Myers January 28, 2026
Authorized generics? More like corporate mind control. Did you know the FDA is owned by the same people who run Pfizer? They let these things through because they want you to think you’re getting a deal. You’re not. You’re being groomed. The real generic? The one made in India? That’s the one you want. Not the ‘authorized’ version. That’s just the brand in a cheap suit.
Praseetha Pn January 29, 2026
Ugh. So the same pharma giants who raised prices 2000% are now ‘generous’? Please. I’m not buying it. I’ve seen the factories. I’ve seen the contracts. They’re not lowering prices-they’re just moving the profit margin from your pocket to their offshore account. And don’t get me started on how PBMs get kickbacks from the brand for pushing the ‘authorized’ version. This is a trap wrapped in a label.
Andrew McLarren January 30, 2026
It is my professional opinion, based upon an analysis of the U.S. Food and Drug Administration’s public database, as well as the Federal Trade Commission’s 2022 report on pharmaceutical market dynamics, that the introduction of authorized generics represents a legally permissible, economically rational, and ethically ambiguous mechanism for price suppression within the context of patent expiration. While consumer outcomes are generally improved, the structural incentives for market manipulation remain intact. Therefore, one must approach this phenomenon with cautious optimism.
Robert Cassidy January 31, 2026
America’s healthcare system is a joke. But at least this? This is a tiny win. I don’t care if it’s Big Pharma’s PR stunt. If I can get my cholesterol meds for $12 instead of $110? I’m taking it. I’m not here to fight the system-I’m here to beat it. And if the brand company wants to sell me the same pill for less so they don’t lose money? Fine. I’ll take their ‘generosity’ and use it to buy groceries.